Live Chat Log for Chemistry
This is our "extra session" for chapter 9 -- I've learned from experience that it usually requires some extra time to get through all the homework.
Hullo.
Hello, Dr Christe!
I think I'm already in vacation mode.
I wish I could say I was in holiday mode....but I've got so much work to do!
:-P
Are there any questions you have about the material in chapter 9 or problems with the homework?
Could we practice more with the Lewis Electron Dot Structures? -- in specific where the dots go?
Good idea, Edmund.
Dots go as far away from other dots as possible.
Did you have something specific in mind?
(I had some trouble with the ones in 29.)
(If Edmund doesn't have something else.)
I'm fine with that, Peter.
OK...So N2H4, PO43-, BH4-, and NH2OH
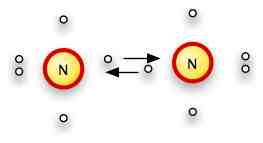
For N2H4, first we draw the Lewis diagram for each atom.
N has 5 valence electrons, H has one:
There are three open places on the N (to bring it up to a full octet), and one on hydrogen to fill its 1s level.
We need three bonds on each of two nitrogens and on on each of 4 hydrogens.
A little thought will show that we can bond the two nitrogens together, then bond two hydrogens to each nitrogen.
H2-N-N-H2
OK?
Yes.
Yes.
Yes.
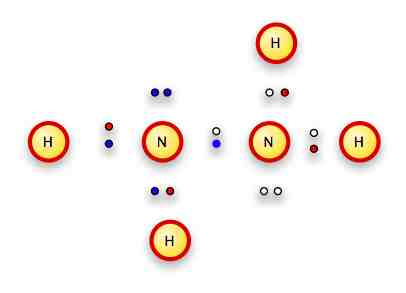
So let's start by putting the nitrogens together.
They have to share an electron, so we'll put the paired sets on opposite sides and have each nitrogen contribute an electron to the bond:
Now we want the hydrogens as far apart as possible because their electrons will be next to the nitrogens, leaving the rest of the atom's positive center unshielded -- so the nuclei of the hydrogens will repel one another.
We also have the electrons in the free pair taking up space.
So two of the hydrogens should go on the end, and the free pairs should go on opposite sides of the molecule.
We wind up with something like this:
I have a question,
Notice that the H's electrons are red, the left nitrogen's electrons are blue, and the right nitrogen's electrons are black.
GA Peter
The books list several steps for drawing L structures,
the 5th is, if the central atom has fewer than 8
to move lone pairs into intermediate positions.
Here, wouldn't that mean moving the extra 4 electrons into intermediate positions?
Between N and H?
(Rather than making them lone pairs on N)
The central atom doesn't have less than 8 electrons at this point.
The central atom(s) are the nitrogens.
Each has 5 electrons of its own, one shared from the other nitrogen, and two shared from each attached hydrogen.
Its octet is full.
The condition of "fewer than 8 electrons" applies AFTER we've put the bonds in place.
Each nitrogen needs three bonds -- and each nitrogen has three bonds.
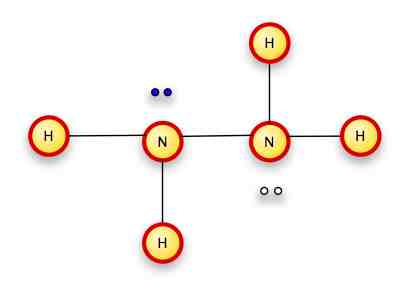
If we replace the "mixed" pairs above (where the two electrons are from different atoms) with bond lines, this is easier to see.
Does that help?
Yes.
Thank you.
If we moved the lone pair between an N and H, the H would have 4 electrons and the N 10 -- that doesn't work.
Any other questions on N2H4?
Nope.
No, I don't.
No.
By the way, this particular molecule is hydrazine.
In 3-dimensions, it looks more like this:
0.0
Our 2-dimensional Lewis diagrams don't do it justice!
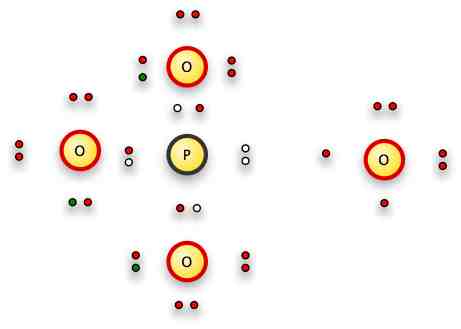
Next: PO43-
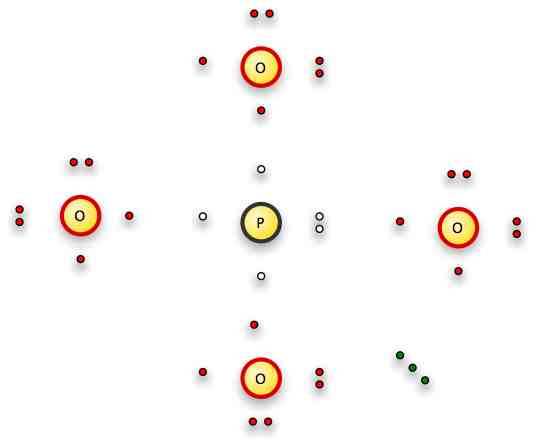
Phosphorus is below nitrogen on the periodic table, so it too will have 5 valence electrons and optimally make three bonds.
Oxygen will try to make two connections where possible -- but here we have an extra three electrons to play with.
So if we push one of the free electrons on 3 of the oxygens, we now have 3 single bonds the oxygens can make with the phosphorus.
So here's what we have to play with:
The three green electrons are our free electrons, represented by the "3-" in the formula.
We'll put one each on three of the oxygens...and then bond those oxygens by single bonds, one oxygen and one nitrogen electron each.
That leaves us with the oxygen on the right -- and no bonding electrons left because the phosphorus has its octet full.
But wait!
One electron is EXACTLY the same as another electron.
We've got a PO33- complex which is very attractive to our oxygen.
Sooooo...it shifts its two singleton electrons into the same orbital and just cosies up to the phosphorous complex
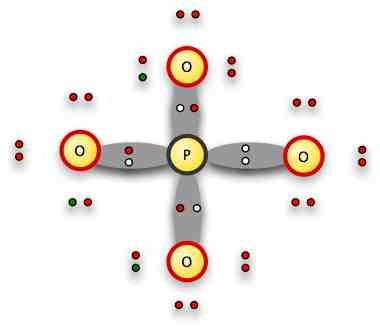
The grey areas represent the bonding electrons.
Questions, or is this starting to make some kind of sense?
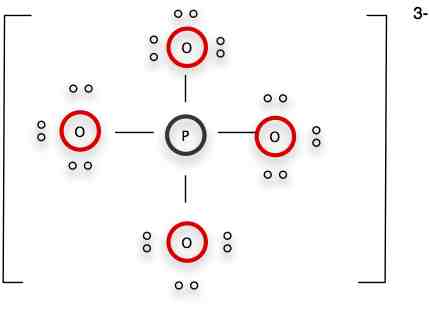
In a formal representation, by the way, you'd put brackets around this to show that it is an ion, and then put the charge on the brackets in the upper right.
What happened to the red dot on the right Oxygen?
I'm a little unclear about the shifting of electrons from one diagram to the next: it looks like you added another electron to the O on the right. Am I missing something?
Nope...I didn't.
If you scroll up, you'll see that the O on the right had six red dots.
Er, sorry, I just realized I made a mistake.
Two pairs, and two singletons.
I moved one of the singletons down to form another free pair, which left a space for the O to share the P's free pair.
Oh, I see!
With its own electrons out of the way in free pairs on the opposite side of the O atom from the phosphorus, the positively charged O nucleus can be attracted to the overall 3- charge of the ion, and share the P's free pair.
Here's the formal notation:
Does that clear it up? if not ASK NOW!
Yes, it does. :-)
Yes.
Masterpieces.
:-)
I just hope they are more enlightening than confusing.
Yes, they are. ;-)
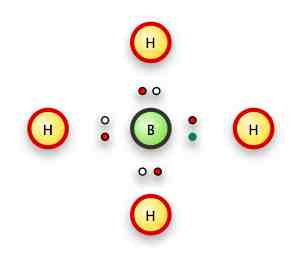
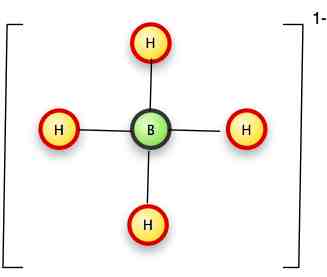
Next we have BH4-
Boron is element #5: two of its 5 electrons are in its 1s level, so it has three valence electrons.
With the addition of the free electron, tho, it becomes similar to carbon with 4 valence electrons.
We can put a hydrogen on each side:
(The green electron is the free electron giving the ion its charge).
Or more formally with lines replacing the bonds, and brackets to show the ionic state:
Any issues with BH4-
No.
No.
I don't.
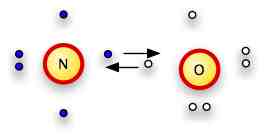
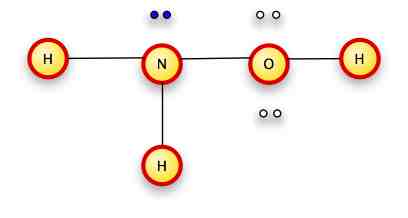
Now...take a close look at NH2OH
It's not that different from N2H4>
We've replaced one of the N's with an O -- which has one more electron than the N, so it has a free pair instead of a place to bond.
We put the N and O together.
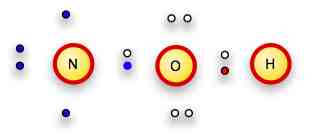
We put ONE H on the Oxygen, since it only has one remaining slot for a bond.
We add the other two electrons to the nitrogen.
err.. other two hydrogens
The nitrogen and oxygen each have 8 electrons surrounding them, the hydrogens each have two pair.
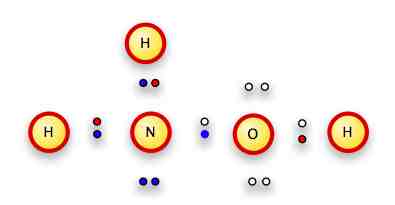
In conventional notation:
(we can put the H above or below the nitrogen; it doesn't matter).
We don't use brackets because this isn't an ion.
Any questions left on #29?
I'm not sure why you place the remaining dots where you do.
I thought you fill terminal atoms first?
I can't put any more electrons on the hydrogens.
They each already have one of their own and one shared -- they are "full" at two (this is true of hydrogen and helium only; the rest can take octets).
Each line represents a single bond of two electrons.
If we count up the bonds around the nitrogen: 3 bonds represent 2 * 3 = 6 electrons.
There is a single pair of the nitrogen's own electrons -- so it has an octet -- 2 in a free electron pair, six in bonds.
Five of those electrons came from its own electron shells, but it sees the shared electrons (one from the oxygen, one from each of two hydrogens) as its "own".
It's kinda like a mom when the neighborhood kids come over to play in her yard.
She keeps an eye on all of them and provides snacks for all of them - they are in her sphere of influence.
So the electrons in the nitrogen's neighborhood contribute to the stability of its electron configuration -- whether or not they originally came from the nitrogen, the hydrogens, the oxygen, or outer space.
From the hydrogen's point of view, it has two electrons which is enough to fill its 1s energy shell, reach stability, and complete hydrogen nirvana.
It doesn't care that one electron came from the nitrogen....they both appear of equal charge value to it.
(I suspect there is a serious theological message there!)
The nitrogen and hydrogen lay claim to the same set of electrons -- the ones in the bond -- and they both hang together.
:-)
Neither is willing to let go of either electron.
Does that help?
Yes.
Yes indeed.
Thank you very much.
Yep, it makes stuff a lot more clearer.
Does that mean the dot positions make better sense now?
Yes, much.
We need two electrons for H and He, 8 for everything else to be in the most stable (lowest energy) position.
Yes, I'm getting a better grip on this material.
So in putting these molecules together the goal is to get some combination of lines (= 2 electrons, representing bonds) or free pairs (2 electrons in each pair) around every atom that isn't hydrogen or helium.
If the molecule needs to swipe electrons from the environment, it will.
You may remember that there is only ONE naturally occuring POSITIVELY charged ion (NH4)+
All of the others are negatively charged -- because there are lots of electrons ready for the swiping (just comb your hair in dry weather).
We've also now had a couple of examples where the net ionic charge on part of a molecule means that one of the atoms doesn't actually contribute to the bond.
Instead, both electrons in the single bond come from the same atom -- and that works. If an arrangement creates a stable octect arrangement for BOTH atoms, it means both are at a lower energy level together than apart -- and nature seeks the stability of low-energy levels.
"Water runs downhill"
Hope that helped.
Yes. It did.
I think we are done for tonight.
Thank you very much.
You're welcome.
Thanks, Dr. Christe!
Thanks for class, Dr. C!
Do a few more Lewis diagrams and check them to see if they really make sense now.
Have a blessed Christmas and a Happy New Year!
You too!
Okay. Have a very merry Christmas!
Well, I hope you have a merry Christmas!
(Happy Christmas, Edmund!)
See you all next year!